COVID Vaccine News and Information (updated 10/16/23)
St. Luke’s will begin administering the 2023-2024 COVID-19 vaccine on Tuesday, Oct. 24 to adults ages 19 years and older. Scheduling will open Oct. 20 in MyChart.
Per the Centers for Disease Control and Prevention, the vaccine is expected to be covered by most insurance plans, including Medicare and Medicaid. Uninsured adult patients can find providers offering free immunizations at Vaccines.gov.
We are not yet administering the updated COVID vaccine to patients ages 6 months through 18 years old, as we are continuing to update our systems. We will begin scheduling and administration for this group as soon as possible.
The updated COVID-19 vaccine formulated for better protection against newer circulating COVID-19 strains was approved in September.
COVID-19 Vaccine Recommended for Pregnant Women
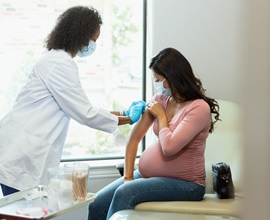
Protect Yourself and Your Baby
Pregnant women with COVID-19 can have severe illness and severe outcomes, including ICU admission, mechanical ventilation, and death.
That's why St. Luke’s OB/GYN providers recommend the COVID-19 vaccine for people who are pregnant, breastfeeding or trying to get pregnant. More than 139,000 pregnant women have enrolled in the V-Safe Pregnancy Registry. Data shows there is no risk of miscarriage associated with the vaccines.
COVID-19 vaccines do not cause infection; they do prevent severe illness from COVID-19, which is dangerous for a pregnant woman and her fetus. Maternal infection with the virus is associated with an increased risk of preterm delivery and stillbirth. Vaccinated mothers who are breastfeeding transmit antibodies that may help protect their newborns.
FAQs: Vaccine Basics
Click each question below for its answer:
Pfizer has reported that its vaccine is 95% effective in preventing COVID-19 infection, once seven days have passed since the second vaccine dose (booster shot). The Moderna vaccine is reporting a 94.5% effectiveness rate.
The COVID vaccine will help your body generate antibodies to help protect you from the virus without getting sick. Vaccines can produce longer-lasting protection than if you had the disease. Research shows antibodies in recovering adults last up to four months. The CDC says more data is needed to know how long immunity produced by the vaccination will last.
In a Pfizer trial study, the vaccine prevented the disease in 100 percent of the 1,131 adolescents who received it. Pfizer said the vaccine was well tolerated and side effects were consistent with those reported by people 16-25 years old. Our pediatricians and physicians say it is better to get the vaccine than to risk getting the disease, the symptoms and the possible long-term effects.
Please review the fact sheets for each vaccine, as developed by the manufacturers:
These are not new technologies. The vaccines are actually set up to give us immunity that is better than natural infection. We know the side effects of the natural infection. When we compare the risks to those at the frontlines that are being exposed to the virus and those in our community who are at high-risk for severe disease and death, those risks greatly overshadow the unknown risks of the vaccine.
No, it is made from a portion of the virus’ molecular material (RNA). For more information on the science behind the vaccine, please visit Facts about COVID-19 Vaccines.
Yes, because the vaccine is 95% effective, not 100% effective. Those exposed to the virus later, after the booster vaccine, tended to have mild symptoms if they became ill at all.
The American Lung Association’s blog says, “In most cases, herd immunity is not achieved without an effective vaccine. For COVID-19, the percentage of the population that needs to be infected to achieve herd immunity is estimated to be between 70% and 90%, and this is assuming lasting immunity is possible.”
Herd immunity could happen naturally but would take years. Also, we know acquiring immunity through natural disease is risky to that person and comes with a high cost of hospitalizations, long-term health problems and even more deaths.
Older age and underlying medical conditions including obesity, a compromised immune system, hypertension, COPD, diabetes, and heart disease increase the risk of severe illness from the virus and should be considered as well. You may wish to discuss with your primary care provider.
Idaho Resources
National Resources
- Centers for Disease Control (CDC): COVID-19 FAQs
- National Institutes of Health (NIH): Coronavirus
- Food and Drug Administration (FDA): COVID-19 Vaccines
FAQs: Getting the Vaccine
Click each question below for its answer:
The COVID-19 vaccines have been available since 2020 and continue to be updated to better fight currently circulating variants. St. Luke’s administers the Pfizer vaccine. Stay up to date with COVID-19 vaccines.
If you are an adult 18+, you can schedule by following these steps:
- Log into St. Luke’s myChart. (If you don’t have an account, you can create one online or by calling 208-381-9000.)
- Click on Schedule COVID Vaccine Appointment.
- Answer a few quick questions and then schedule your vaccine appointment.
- Minors cannot consent for a COVID vaccine. They must be accompanied by a parent or legal guardian to provide consent at the time of the appointment. Written or verbal consent will need to be provided by a parent or legal guardian, if not present at the time of the appointment.
- Teens 16-17 years old can only receive the Pfizer vaccine, which is available at St. Luke’s sites in Boise, Hailey, McCall, Meridian, Mountain Home, Nampa and Twin Falls.
- Minors cannot schedule their own appointments in myChart. Parents or legal guardians with teen proxy access can schedule for the minor. To get teen proxy access, the teen will need to grant teen proxy access to their parent or legal guardian through their own MyChart account.
- Parents can schedule without proxy access by calling 208-381-9500.
- Minors can schedule their own vaccine appointment by calling 208-381-9500, however, they must be accompanied by a parent or legal guardian to provide consent at the time of the appointment, or provide written or verbal consent of a parent or legal guardian, if not present at the time of the appointment.
How to Schedule
- Log into St. Luke’s myChart. (If a minor doesn’t have an account, they will need to get an activation code online or by calling 208-381-9000.)
- Click on Schedule COVID Vaccine Appointment.
- Answer a few quick questions and then schedule your vaccine appointment.
- Or call 208-381-9500.
- Minors cannot consent for a COVID vaccine. A parent or legal guardian should accompany minors to provide consent at the time of the appointment. Written or verbal consent by phone may be accepted if a parent or legal guardian is not present.
- Children 12-15 years old can only receive the Pfizer vaccine, which is available at St. Luke’s sites in Boise, Hailey, McCall, Meridian, Mountain Home, Nampa and Twin Falls.
- Minors cannot schedule their own appointments in myChart. Parents or legal guardians with teen proxy access can schedule for the minor. To get teen proxy access, the teen will need to grant teen proxy access to their parent or legal guardian through their own MyChart account.
- Parents and children can walk in or a parent can schedule by calling 208-381-9500. Note: we are not able to accommodate walk-ins in Hailey.
- Patients should wear loose-fitting clothing to allow access to the upper arm.
- Parents should be aware that we will not give the vaccine to children who don’t want it.
- Minors cannot consent for a COVID vaccine. A parent or legal guardian should accompany minors to provide consent at the time of the appointment. Written or verbal consent by phone may be accepted if a parent or legal guardian is not present.
- Children 5-11 years old can only receive the Pfizer vaccine at some St. Luke’s sites. Find a list of available sites and appointments at the time of scheduling.
- Minors cannot schedule their own appointments in MyChart. Parents or legal guardians with proxy access can schedule for the minor.
- At this time, we are not able to accommodate walk-in appointments for this age group.
- Patients should wear loose-fitting clothing to allow access to the upper arm.
- Parents should be aware that we will not give the vaccine to children who don’t want it.
Please review our visitor policy.
Pfizer and Moderna: The most common side effect is injection site pain. Other side effects of the Pfizer and Moderna vaccines include fatigue, headache, muscle pain and chills, and generally last two days before subsiding. These side effects indicate that the vaccine is doing its job, mimicking an infection in the body without causing a COVID-19 infection. Severe adverse reactions such as allergic reactions and Bell’s Palsy are much less common, but if they occur, need to be reported to your health care provider. None of these side effects are contagious.
It is important to note that reactions after the vaccine and the actual COVID-19 infection are significantly different. Vaccine reactions may involve some mild symptoms occurring in the first couple of days, coupled with the pain at the injection site, redness and swelling (from the vaccine), as noted above. By contrast, the COVID-19 infection reaction has a respiratory component, cough and nasal congestion, loss of taste or smell, shortness of breath, much more fever, and also a longer period of muscle pain, fatigue and headache.
Infrequently, people who have received dermal fillers may develop swelling at or near the filler injection site, usually face or lips, after receiving a dose of an mRNA COVID-19 vaccine (Pfizer or Moderna). It appears to be temporary and can resolve with medical treatment, including corticosteroid therapy. People who have received dermal fillers can be vaccinated without additional precautions but should contact their health care provider if they develop swelling afterward.
Yes, the CDC has updated their guidance to state that health workers may administer another needed vaccine at the same time as the COVID vaccine. This applies to patients of any age.
If the person has recently received the COVID-19 vaccine, the current CDC recommendations state the need to defer TST (TB skin test) or IGRA (TB blood test) until four weeks after completion of the second dose of COVID-19 vaccine. If the person is in that COVID-19 vaccine window, we recommend a healthcare screening to ensure that an individual does not have any symptoms of active (contagious) tuberculosis disease. This screening is provided by the Occupational Health clinics.
This health care screening does not rule out latent tuberculosis, which is when the disease is only in a dormant state and cannot be spread to others. However, with this preliminary clearance, testing for latent TB can be deferred until the four-week post-COVID-19 booster waiting period has ended. Once that person is past their COVID-19 vaccine window, they should proceed with the IGRA or TST, screening for latent TB.
FAQs: After Vaccination
Click each question below for its answer:
The CDC has published information on What to Expect after Getting a COVID-19 Vaccine and posts updates as more information becomes available.
When you receive a vaccination in one of our clinics, you will have the option to be monitored for about 15 minutes and medical staff will use safety precautions and respond immediately if you have an initial reaction. If you have a delayed reaction, please contact your primary care provider or call 911.
It is most safe to visit those who are also vaccinated. The COVID-19 vaccines are not 100% effective and some, such as those who are unable to be vaccinated (e.g., young children) and some immunocompromised people who may not have developed robust antibodies to the vaccine, may be less protected and/or not protected.
Getting the COVID-19 vaccine will affect when or if you are eligible to donate blood. View Red Cross guidelines